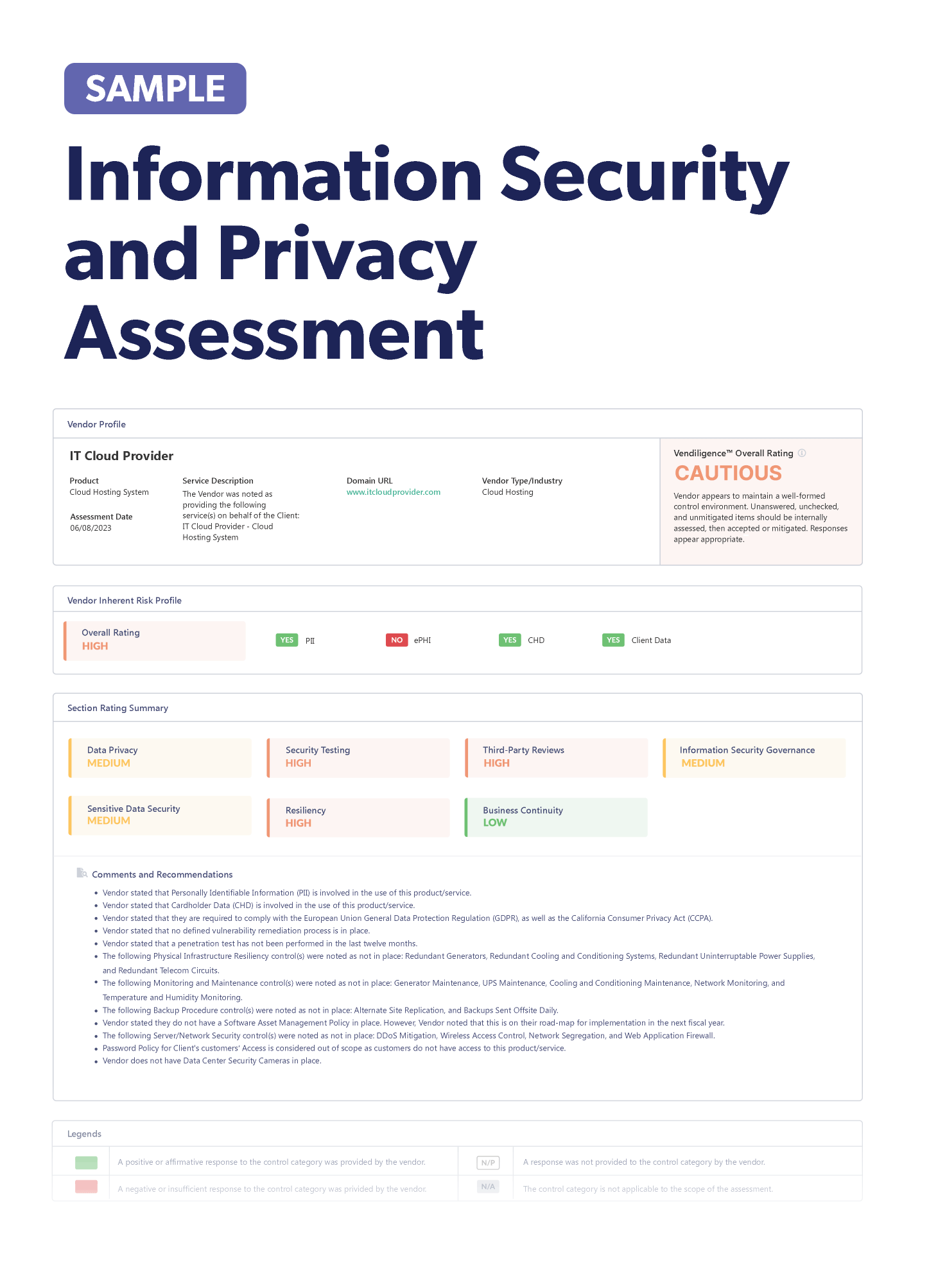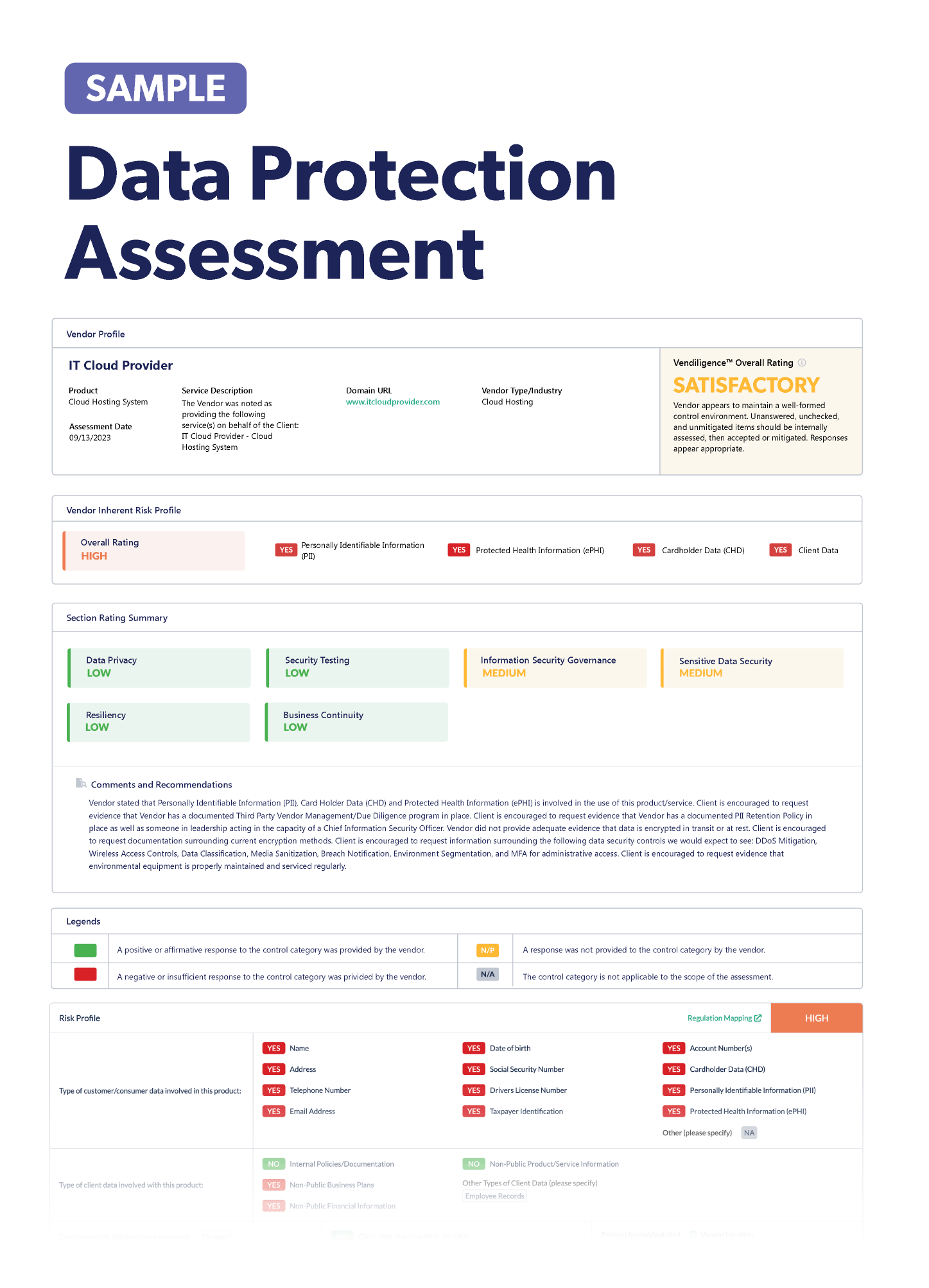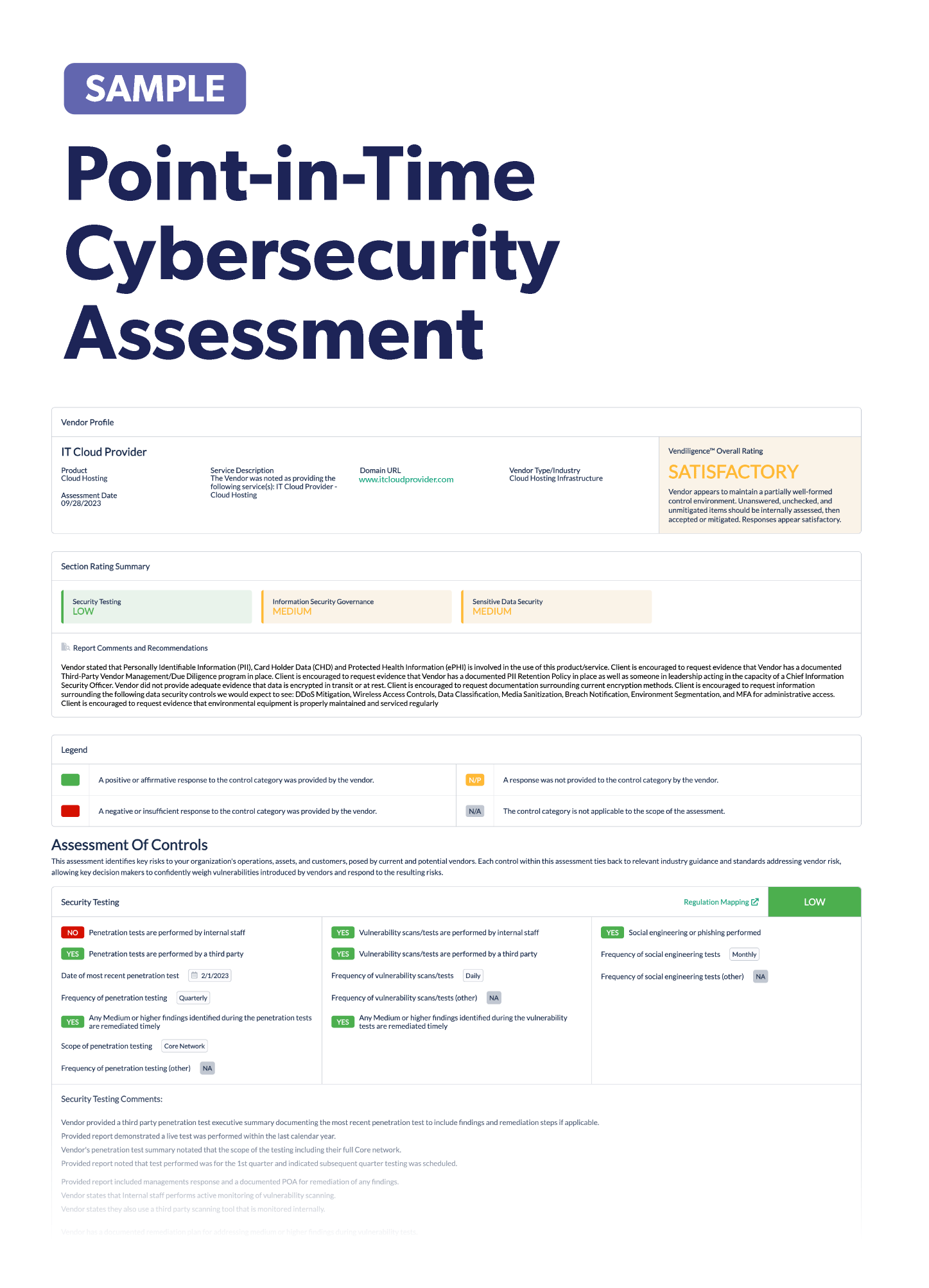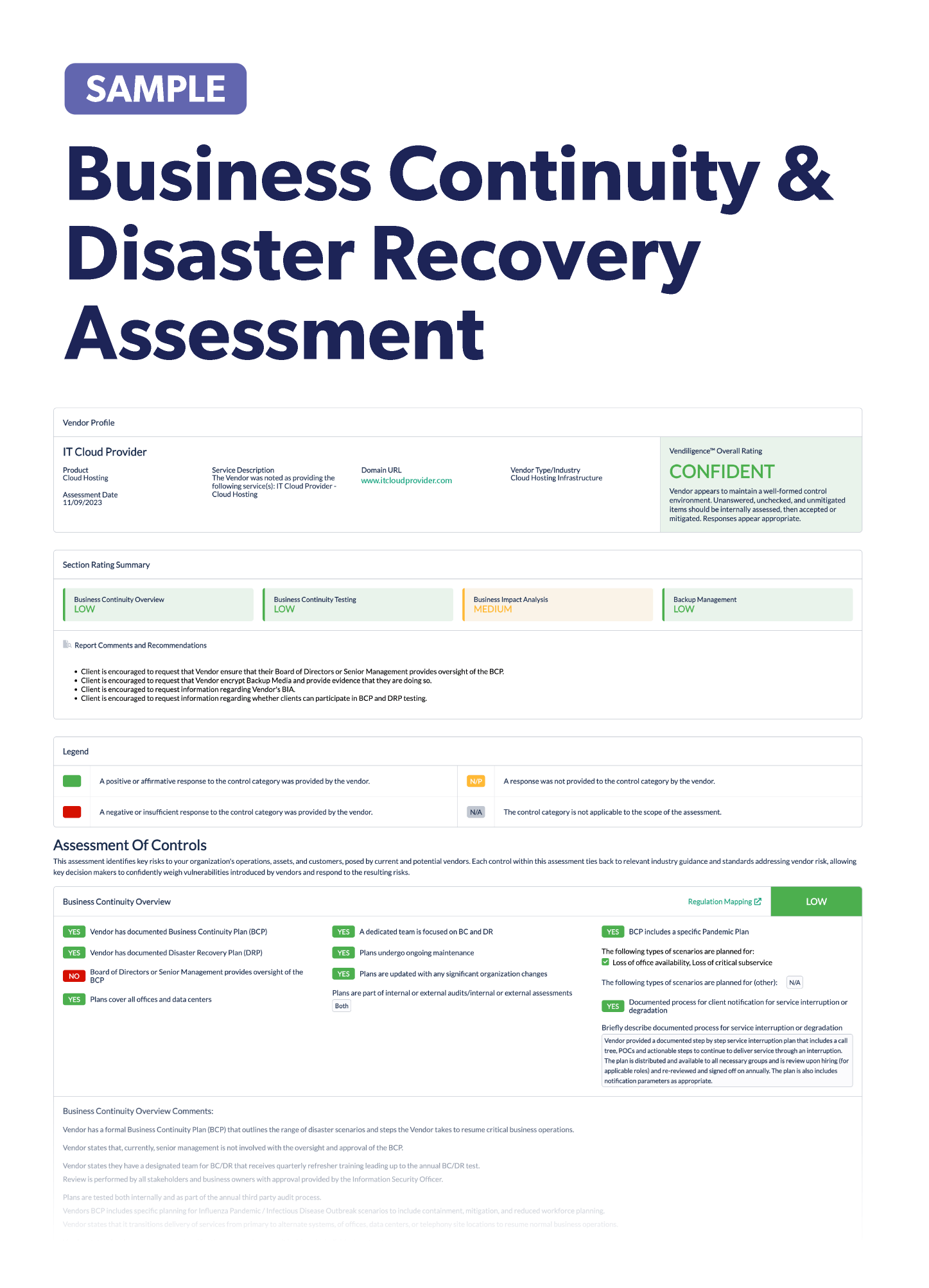
Vendor Risk Management in the Pharmaceutical Industry
By: Venminder Experts on August 7 2024
7 min read

The pharmaceutical industry is a trillion-dollar global sector that comprises an incredible range of organizations, from large, multinational corporations to small, specialized firms. These organizations conduct in-depth research, develop innovative medications, and manufacture, produce, and establish essential medicines and healthcare products worldwide.
The pharmaceutical industry's products directly impact human health, making it a top priority to ensure their safety, efficacy, and high quality. However, a pharmaceutical organization's relationships with its vendors, suppliers, and distributors can pose a broad spectrum of risks.
These risks must be carefully managed at every stage, from research and development to the use of medications and even during monitoring of their safety and effectiveness after consumption. Vendor risk management (VRM) is essential in the pharmaceutical industry due to the numerous potential risks and organizations involved.
Let’s explore how vendor risk management is crucial for pharmaceutical organizations in managing risks.
Vendor Risks in the Pharmaceutical Industry
Vendors can pose a number of risks to the pharmaceutical industry. These risks can disrupt operations, cause financial damage, or lead to regulatory action.
Here are some of the vendor risks in the pharmaceutical industry:
- Regulatory compliance risk: The pharmaceutical industry is bound by stringent regulations, such as the Food and Drug Administration's Good Manufacturing Practices (21 CFR Part 10) and Good Distribution Practices (21 CFR Part 211). Beyond the U.S., the European Medical Agency (EMA) and the World Health Organization (WHO) also play critical roles in regulation, including reviewing and approving new drugs, monitoring the safety of approved drugs, and enforcing regulations on drug manufacturing and marketing.
Whether your organization is responsible for research or development, providing raw materials and components, drug manufacturing, packaging, and labeling or distribution of these medications, you must be sure that all your vendors, suppliers, and distributors are rigorously compliant with all applicable regulations for all relevant jurisdictions. Regulatory failures anywhere within the supply chain can impact drug safety and effectiveness or result in drug recalls or removal from the market altogether. - Operational risk: The pharmaceutical industry relies on an intricate global supply chain involving numerous suppliers, manufacturers, and distributors. Pharmaceutical organizations must have complete visibility into their suppliers and suppliers' suppliers to mitigate risks. Understanding the entire supply chain and identifying potential weaknesses at every stage is crucial. Without the proper precautions, a single supplier could disrupt or shut down an entire supply chain.
These examples help you further understand operational vendor risk in the pharmaceutical industry:
- A cyberattack against a SaaS inventory management system creates a domino effect that impacts materials management and purchasing.
- An unplanned factory closure in one region impacts production schedules in another.
- The "cold chain" for temperature-controlled vaccines and medications is broken when the distributor doesn’t have contingency plans for a prolonged power outage due to a natural disaster.
- Geopolitical risk: The pharmaceutical industry heavily relies on a global network of vendors for the procurement of raw materials and the execution of manufacturing, packaging, and distribution processes. Manufacturing activities in regions characterized by relaxed regulations and limited oversight amplify the susceptibility to counterfeit drug production and human rights violations.
Geopolitical uncertainties, including political unrest, regional tensions, and public demonstrations, can disrupt supply chains, leading to shortages and delays. Additionally, the imposition of import tariffs or export bans may significantly affect material availability, impacting the movement of pharmaceutical ingredients, finished products, or causing pricing fluctuations. - Financial risk: Unmanaged vendor risks can result in severe economic losses. Drugs pulled from the market due to manufacturing errors, contamination, or other safety concerns impact a pharmaceutical organization’s reputation and revenue. Investments made in research and development may be forfeited due to a lab's questionable regulatory compliance or testing protocols.
The decision to hastily move production from one country to another due to political unrest can result in unplanned increased labor costs and product shortages. However, the financial impacts aren't just limited to production costs and revenue loss; the costs of defending sizeable litigation and paying legal damages are another example of financial losses that occur due to insufficient vendor risk management.

Managing Vendors in the Pharmaceutical Industry With the VRM Lifecycle
Vendor risk management is a framework of processes designed to systematically identify, assess, mitigate, and monitor the myriad of risks posed by an organization's dependence on external research and development organizations, producers and suppliers, manufacturers, distributors, and subcontractors. Vendor risk management is a discipline often embedded into the supply chain management (SCM) function and is complementary to other SCM processes, such as sourcing and procurement, demand planning and inventory management, logistics, quality control, sustainability, and information management.
Vendor risk management involves a set of defined activities and processes assigned to a fixed lifecycle that includes three stages: onboarding, ongoing, and offboarding. To ensure maximum risk management effectiveness, the activities and processes must occur in a specific order and at a predictable frequency.
Let's explore the activities and lifecycle stages for vendor risk management in the pharmaceutical industry:
Onboarding
During the onboarding stage, the pharmaceutical organization plans for the relationship, identifies the risks involved, verifies the vendor's risk management practices and controls, manages known issues, and prepares for and executes the contract.
Here are some of activities included in the onboarding stage:
- Planning: The planning activity sets the stage for the eventual third-party relationship and can include:
- Defining the goals and objectives for the product or service and its relationship within the supply chain.
- Assigning internal responsibility for the specific vendor and managing the risks associated with the relationship as a vendor owner, third-party vendor manager, or relationship manager.
- Sending RFPs or RFIs to gather essential information about potential vendors, such as capabilities, product offerings, pricing structure, use cases, project solutions, and other details necessary to shortlist potential suppliers or award business.
- Identifying an exit strategy and plan to detail the organization's approach in case the vendor relationship, product, or service is no longer viable. Strategies include switching to another vendor, bringing the product or service in-house, or discontinuing it altogether. Exit plans provide a detailed overview of how to execute the strategy and consider the return or destruction of data, return or transfer of assets or inventories, roles and responsibilities of all parties, contingencies, etc.
- Risk Assessment: An inherent risk assessment identifies the types and amounts of risks associated with the product or service and the vendor relationship. This results in a risk rating or tier that informs all subsequent risk management activities, their frequency, and the scope of due diligence. Keep in mind:
- Determining criticality is essential to identify the vendors, third-party suppliers, providers, producers, or distributors that are critical to the supply chain. Sole source producers of raw materials, for example, can shut down the entire drug production should they go out of business. A cyberattack against an inventory planning system can disrupt production and distribution for weeks or months. While all vendors in the supply chain play an essential role, some are so essential to your operations that the organization can’t continue without them. Identifying these specific relationships as critical helps the organization better manage the risks.
- Due Diligence: A multi-part process that includes gathering and assessing information to ensure vendor risk management practices and controls are in place and sufficient to address identified risks, including:
- Vendor risk questionnaires are completed by the vendor to gather detailed information about the vendor’s risk management practices and controls.
- Due diligence documentation provides evidence of the control environment, risk management practices, business continuity planning and testing, financial health, and more.
- Vendor risk reviews are conducted by subject matter experts (SMEs) to review and assess the information and documentation provided by the vendor. The SME gives a qualified opinion on the appropriateness and sufficiency of the vendor’s control environment within a specific risk domain, such as compliance, cybersecurity, business continuity and disaster recovery, geopolitical, financial, etc.
- Contracting: The process to formalize all requirements, service level agreements (SLAs), and performance metrics. Contracting also secures the vendor’s commitment to meeting the contract's legal terms, conditions, and obligations.
Ongoing
During the ongoing stage, the risk and performance of the vendor are assessed and monitored until the relationship ends and includes:
- Periodic Risk Re-Assessment and Due Diligence: This process ensures new and emerging risks are identified, existing risks haven’t changed, and controls and risk management practices are sufficient and haven’t degraded or failed over time.
- Monitoring and Performance: Ongoing monitoring and management of a vendor’s risk profile helps identify areas of concern, manage issues, and respond to increasing threats. Performance monitoring verifies that vendors meet contractual requirements, maintain quality, and deliver on time. It also monitors trends to identify potential trouble spots requiring remediation or improvement.
Issue management is a key part of monitoring, as it ensures all vendor issues are documented, tracked, reported, and mitigated on time. - Renewals: Contract management ensures your pharmaceutical organization has enough time to consider, renegotiate, or end the contract.
Offboarding
When vendor relationships end due to predictable or unpredictable circumstances, offboarding risks are managed with the following activities:
- Termination: This is an official notification of termination per the contract obligations and timing.
- Exit Plan Execution: This ensures a safe and orderly transition out of the vendor relationship.
- TPRM Closure: Ensures all administrative details, review, and payment of final invoices, and updating of vendor status and eligibility for future opportunities are completed. All vendor records should be organized and appropriately stored.
Whether vendor risk management is a standalone function or embedded within supply chain management, identifying, assessing, managing, and monitoring vendor risks across the supply chain is essential in the pharmaceutical industry. Effective vendor risk management provides the necessary framework and controls to help organizations identify and understand their vendor risk exposure and manage it appropriately.
Pharmaceutical organizations that establish a defined vendor risk management framework and roles and responsibilities, including oversight and reporting, will quickly realize how it aids and supports the efficiency and effectiveness of other essential SCM functions, including sourcing and procurement, compliance, demand planning management, and more.
Related Posts
Manage Geopolitical Risk Energy Industry Third-Party Risk Management
The energy sector is increasingly prone to geopolitical risks. The war in Ukraine, economic...
February 2025 Vendor Management News
Stay up to date on the latest vendor risk management news happening this month. Check out the...
Vendor Risk Management Requirements of NERC CIP-013-1
Energy organizations rely on complex supply chains worldwide, which can expose them to third-party...
Subscribe to Venminder
Get expert insights straight to your inbox.
Ready to Get Started?
Schedule a personalized solution demonstration to see if Venminder is a fit for you.